|
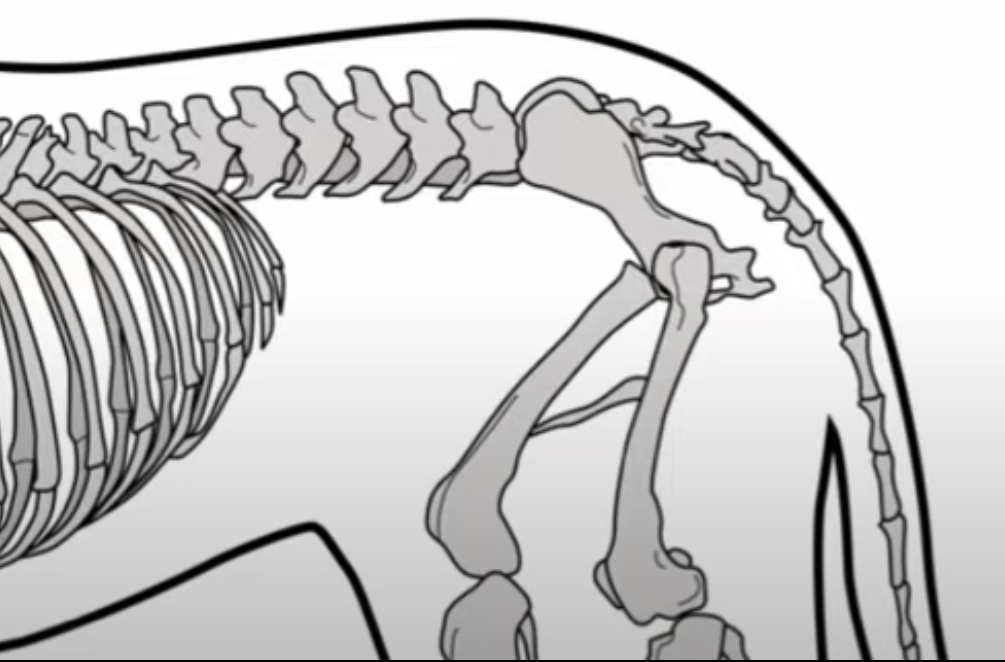
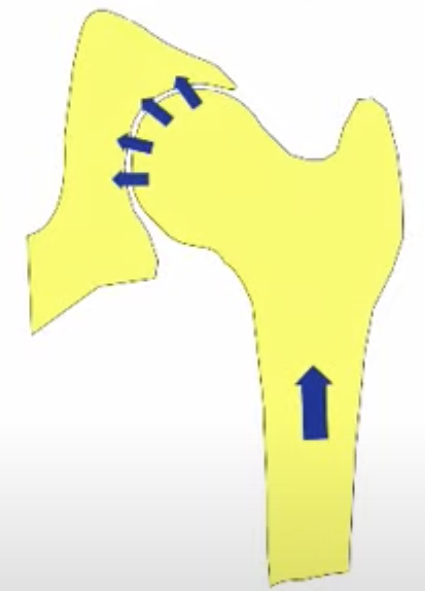
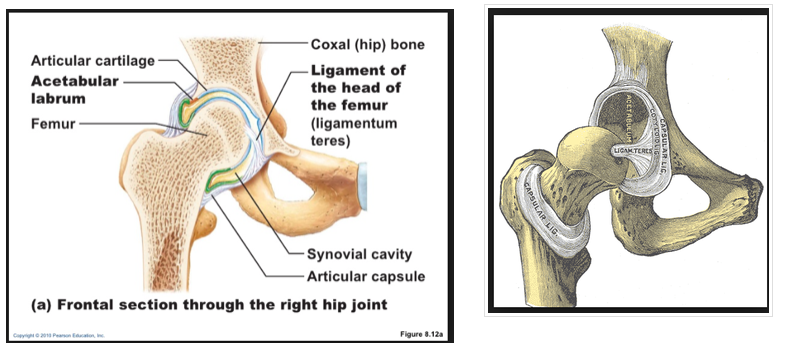
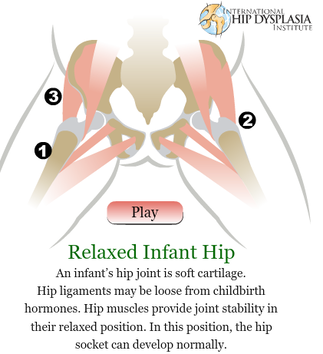
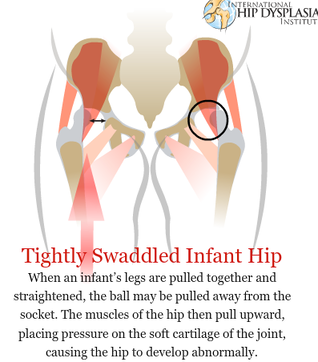
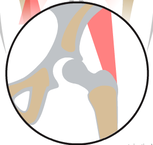
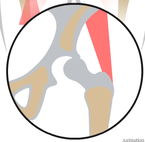
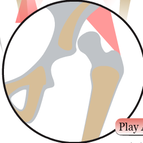
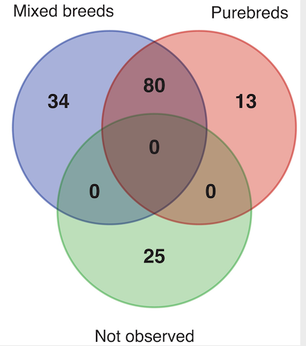
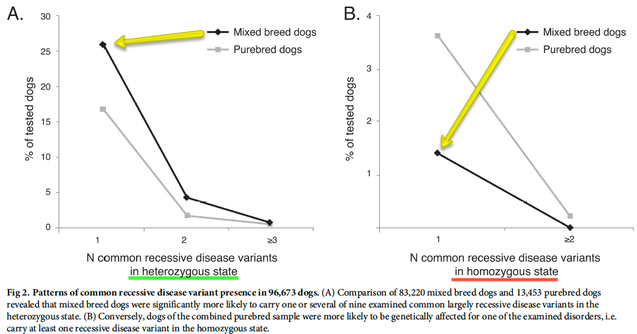
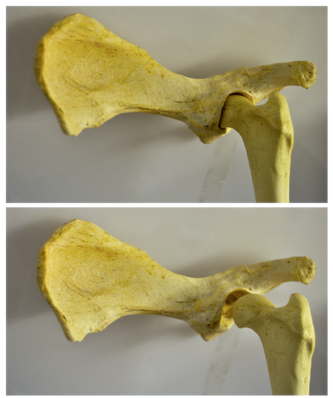
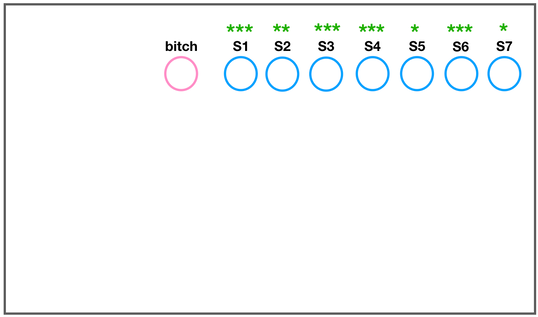
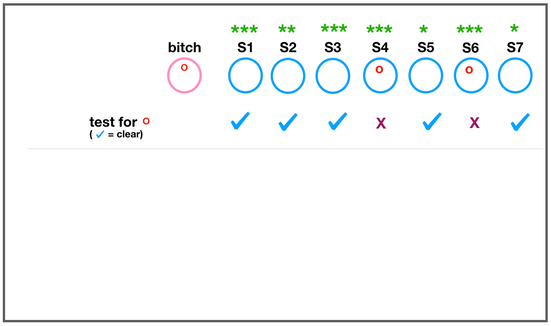
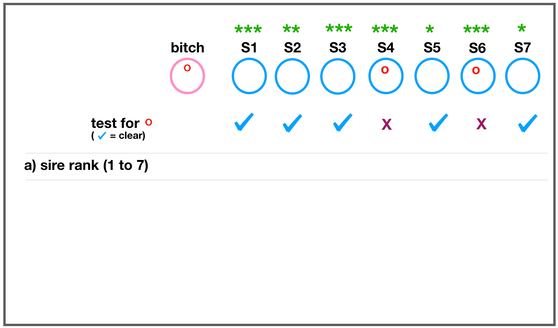
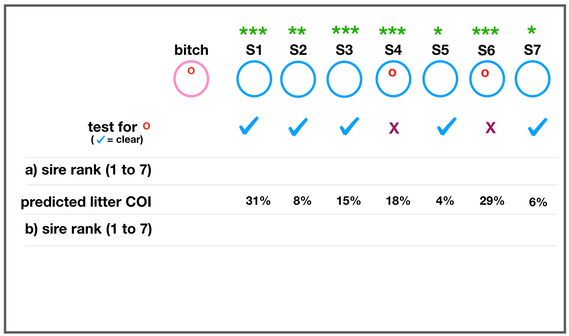
The development of the joint is the result of biomechanical forces, so the correct position of the head of the femur in the socket is critical. The deformity of hip dysplasia is not caused by a "hip dysplasia gene"; it is the result of abnormal biomechanics when the ball is not properly positioned in the socket. This is what Riser is talking about when he says that the hip will develop normally as long as the joint is "coherent", with the ball seated deeply in the socket.
|
*** Visit our Facebook pages ***
ICB Institute of Canine Biology
...the latest canine news and research
ICB Breeding for the Future
...the science of animal breeding