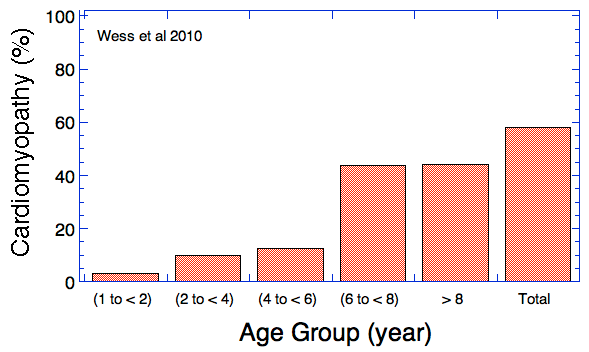
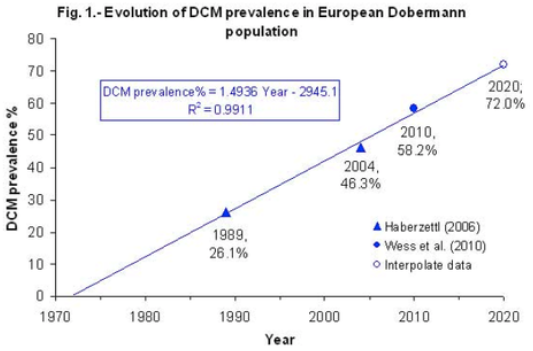
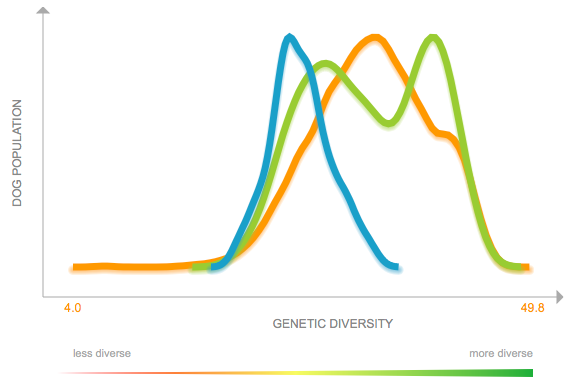
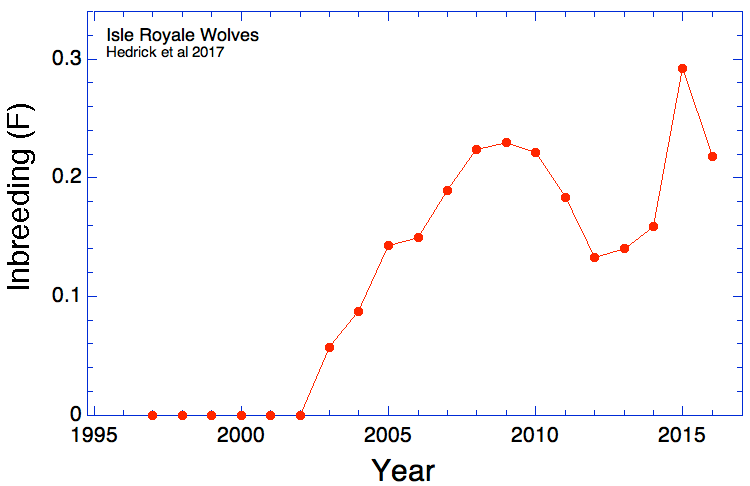
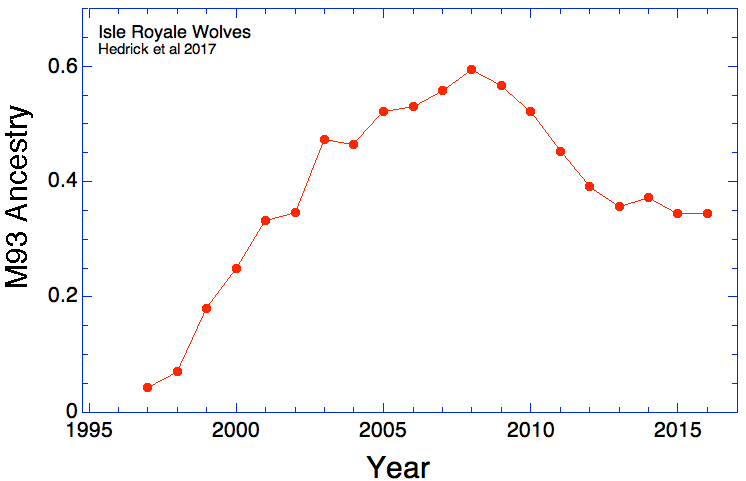
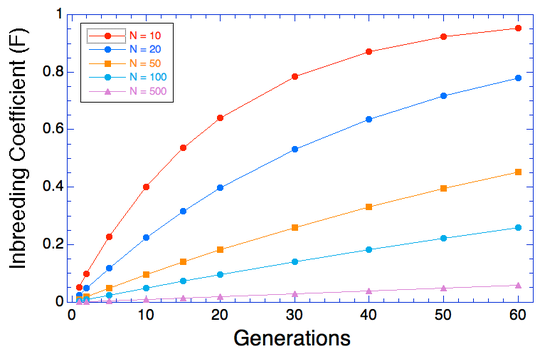
(In the first graph below, the data are divided into age cohorts and no animal appears in more than one age group.)
We have good data about the history of DCM in the breed. In 1990, the incidence was already quite high, with more than 25% of dogs affected. Since then, DCM has increased essentially linearly by about 1.5% per year. At this rate, by 2040, 100% of the Doberman breed will be afflicted with DCM.
Clearly, the efforts made by breeders over the last three decades to decrease the incidence DCM have had no effect at all on the prevalence of the disorder. Not even a little.
What are we doing to deal with this problem? Breeders are routinely monitoring their dogs for the electrical abnormalities that are signs of DCM. They are trying to select from lines that appear to be less afflicted with the problem.
However, the question nobody seems to be asking is whether it is even possible to rid the breed of this problem through selective breeding. Can better monitoring and ever more selective breeding reduce the incidence of this horrible problem in Dobermans? Is there enough genetic diversity in the breed to "breed away" from DCM?
What is the genetic status of the Doberman?
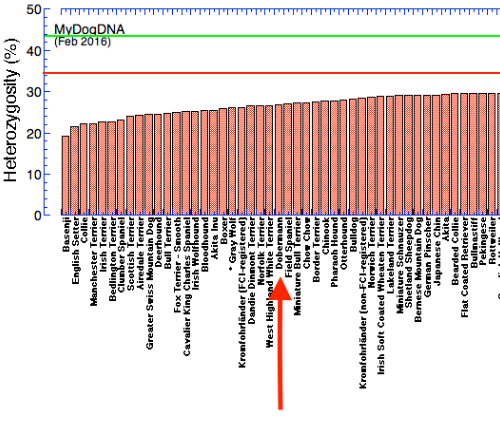
First, this graph is the up-to-date summary of the genetic diversity in Dobermans that have been examined by MyDogDNA. The color scale at the bottom indicates the ranges of high and low diversity. The data include dogs from the US, Austria, Russia, the United Kingdom, Finland, Australia, and Ukraine. The median heterozygosity for this group is 26.6% (blue), which is less than the median for other pinscher and schnauzer-type breeds (34.2%; green) and for all dogs (34.6%; orange).
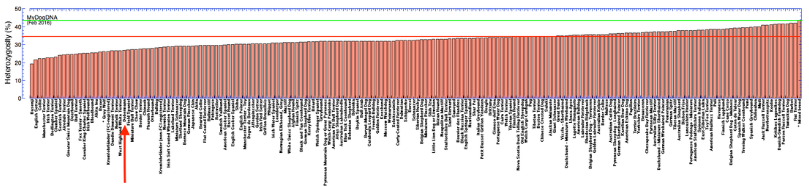
Below I have graphed the data for genetic diversity of all dog breeds analyzed by MyDogDNA on their website. In the top panel, the Doberman, with a median heterozygosity of 26.6%, is indicated by the arrow. Below that is a graph that includes all of the breeds they have measured. (These graphs are taken from my earlier post.) For reference, the green line is the average heterozygosity for mixed breed dogs (43%), and the red line is the median heterozygosity for all dogs in the MyDogDNA database. Higher heterozygosity is better; if a dog was heterozygous at all loci the value would be 50% using this method.
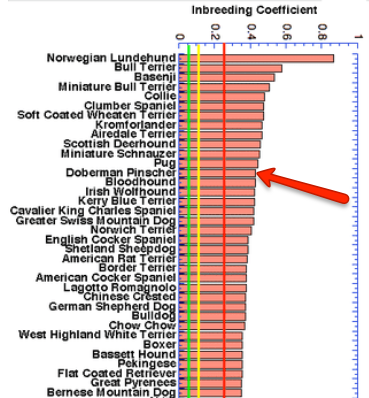
The hand thing about this estimate of inbreeding is that the value indicates two things: 1) the probability that an animal will inherit two copies of the same allele from an ancestor (i.e., homozygous for that allele), and 2) the fraction of all loci that are homozygous.
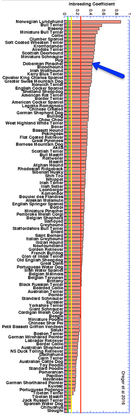
From this study, the average inbreeding coefficient of the Doberman is 43% (red arrow on the enlarged graph on the left, blue on the graph for all breeds on the left). This means that
- on average, nearly half the genome of a dog is homozogyous, with two copies of the same allele;
- the risk of any particular locus being homozygous for the same allele - whether good or bad - is 43%;
- on average, 43% of the genomes of any two dogs are the same.
In addition to the data on inbreeding and heterozygosity of the whole genome in Dobermans, we now also have information specifically about the genes of the immune system.
The immune system protects an animal from all manner of outside invaders, from bacteria and viruses to fungi and parasites. It must be able to recognize a bewildering diversity of pathogens as foreign, then marshall the specific cellular defense mechanisms necessary to destroy them. At the same time, it must be able to distinguish "self" from "non-self"; failure to do this is the cause of autoimmune disorders, in which the immune system attacks one of the body's own tissues.
The genes for the immune system in the dog are called the "dog leukocyte antigens" (DLA). They tend to be inherited as blocks of genes called haplotypes. There are two types of DLA haplotypes called DLA Class I and DLA Class II. Like single alleles, an animal can inherit two copies of the same haplotype or they can be different (i.e., homozygous or heterozygous).
In most animals, the genes of the immune system are the most diverse in the entire genome, and in wild animals there is strong selection to keep them that way. In purebred dogs, however, inbreeding, strong selection, bottlenecks, and genetic drift have reduced the genetic diversity across the genome, including the Class I and Class II DLA.
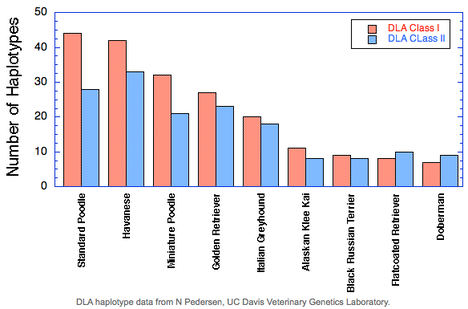
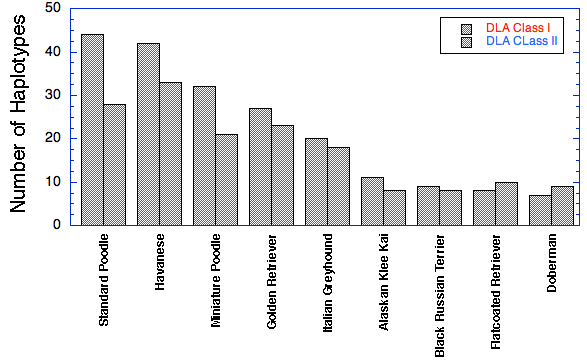
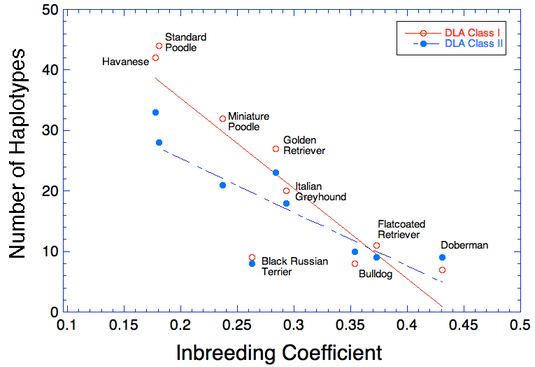
Take for example these data for DLA diversity from UC Davis in nine breeds of dogs (see Inbreeding and the Immune System: Unintended Consequences). The graph shows the number of haplotypes found in a survey of each breed, separated into Class I and Class II DLA.
You can see that the breed with the highest number of haplotypes, the Standard Poodle, has about 45 Class I haplotypes and 28 of Class II. The Poodle suffers from many autoimmune disorders, including Addison's disease, sebaceous adenitis, immune mediated hemolytic anemia, immune mediated thrombocytopenia, chronic hepatitis, temporalmandibular myositis, Evan's syndrome, immune pancytopenia, and chronic thyroiditis (Pedersen et al 2015). Even as it has the highest DLA diversity of the particular breeds in this study, it suffers from a compromised immune system as a consequence of strong selective breeding and genetic bottlenecks (Pedersen et al 2015).
Unfortunately, among the other breeds that were tested, the Doberman fared the worst, with less than 10 DLA haplotypes for either Class I and Class II. Even if DCM could be eliminated from the breed, the Doberman would still suffer from issues realated to the poor health of the immune system.
| 1) "This study of 71 Doberman establishes a desperate need for breeders to search the world for pockets of genetic diversity that does not exist in the present population, just as was done by Standard Poodle and Italian Greyhound breeders. Eastern Europe and more isolated areas of Western Europe would be ideal places to search for such diversity. Genetic introgressions <cross-breeding> with similar dogs may be required, but such outcrossing must be based on sound genetic knowledge and careful monitoring of new diversity to see that it is not lost by backcrossing or contained to only a fraction of the breed." 2) "In the case of diseases such as DCM, the genetic traits responsible for the disease may already be fixed in certain varieties of the breed, reminiscent of hyperuricosuria in the Dalmatian. A lack of genetic diversity greatly limits the ability to find reasonably unrelated mates, but when this lack is combined with the need to select against a large number of heritable traits, the ability to identify genetically suitable mates becomes even more difficult." |
The bottom line
The Doberman has the lowest diversity in the DLA genes of the immune system of any of the breeds studied to date by Pedersen's lab at UC Davis. This, together with the high level of inbreeding documented from multiple studes and the overall relatedness of the dogs in the population, leaves breeders with little ability to circumvent the multiple genetic diseases in the breed. Furthermore, some deleterious genes could be fixed in the breed - that is, the normal, non-mutated version of the gene is no longer present in the gene pool and therefore are not available for selection.
The only hope for this breed is the initiation of a sound, comprehensive cross-breeding program, under the guidance of population geneticists, that will introduce new genetic diversity into the breed. The longer it takes to begin genetic rescue, the more difficult it will be and the less likely it is to be successful.
A final word
We have a moral obligation to restore the noble Doberman breed back to health, and this effort needs to begin immediately.
I repeat what I said in my earlier post:
DNA testing does not make someone a "responsible" breeder. Caring for the heritage of your breed does not make you a "preservation" breeder. Pride and love and dedication are all terrific, but they will not prevent the heartbreak that awaits thousands of Doberman owners in the future. Breeders need to DO something about this. We need to step up to the plate and acknowledge that continuing to breed dogs that are likely to die of a genetic disorder is irresponsible, unethical, and inhumane. That is certainly how the average, everyday dog lover feels. This is also how I feel.
Pedersen NC, L Brucker, NG Tessier, H Liu, M Cecilia, T Penedo, S Hughes, A Oberbauer, & B Sacks. The effect of genetic bottlenecks and inbreeding on the incidence of two major autoimmune diseases in Standard Poodles, sebaceous adenitis and Addison's disease. Canine Genetics and Epidemiology 2:13. DOI 10.1186/s40575-015-0026-5.
Beuchat C. 2016. Are we watching the extinction of a breed? (or, Why are we focused on consequence instead of cause?)
Beuchat C. 2016. Are we watching the extinction of a breed? (part 2)
Beuchat C. 2017 Are preservation breeders preserving the Doberman? (No.)
ICB's online courses
*******************
Join our Facebook Group
ICB Breeding for the Future
...the science of dog breeding
*******************
Visit our Facebook Page
ICB Institute of Canine Biology
...the latest canine news and research