While this took some of the mystery out of breeding for many traits, for other traits prediction remained elusive, and breeders realized that in some cases the genetics of a trait must be more complex. The problem, of course, was that some traits are determined by many genes. How could breeders do selective breeding on these so-called polygenic traits to reliably produce particular traits?
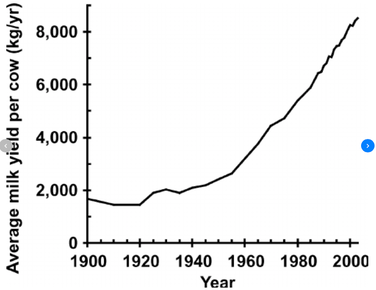
For instance, there could be a range of size in a population of cows from small to large and everything in between, and not just two (binary) sizes of large and small. He realized that this was the case for many traits and began to think about how genetics could account for this. What Lush had stumbled upon was the key to selective breeding for traits that can vary continuously - genetic variation.
He described this in his groundbreaking book, Animal Breeding Plans.
| "Variation - differences between individuals - is the raw material on which the breeder works. It is not necessary that the animals vary widely enough that the breeder can at the very start find some perfect ones to select, but they must vary enough that some of them will be closer to his ideal than others are... The effects of a combination of genes in the individual equal the sum of the average effects of those genes." (Lush 1937) |
This is what he is alluding to when he says that it's not necessary for a breeder to find the exact traits desired in a potential breeding animal, because genetic variation can be used to create a new mix of genes that will produce a trait not present in the current population.
There was one critical thing that was absolutely necessary for this to work: there needed to be genetic variation in the population. Breeders had to protect the genetic variation in their breeding stock, because without it new combinations of genes would not be possible and there would be nothing to select for.
The realization that selective breeding could produce animals that were superior to both parents revolutionized animal breeding. The use of additive genetic variation to produce animals with traits not present in the original breeding stock became the fundamental process of selective breeding in domestic animals.
Body size is an obvious one. Researchers have identified multiple genes that can be associated with variation in body size in dogs. Some of these have a large effect, accounting for a significant fraction of the variation in size. But there is still much variation that is not yet accounted for genetically, and there might be dozens or even hundreds of other genes that have tiny effects individually but collectively account for differences among individuals.
People probably started racing dogs thousands of years ago, and it's fair to suspect that over this time there has been selection to improve speed.
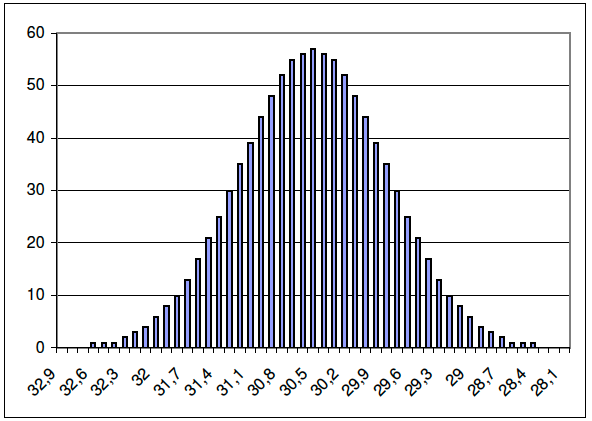
| These are data for 1,000 racing dogs in Ireland over a distance of 480 meters (Taubert & Agena). Most dogs cover the distance in 29-30 seconds. But there is also much variation among individuals, from the slowest that took about 32.5 seconds, to the fastest at about 28 seconds. There could be many reasons for this variation, including motivation, condition of the track on the day, and other enviromental factors. But at least some of this variation might be attributed to genetics. How could breeders use selection to breed faster dogs? |
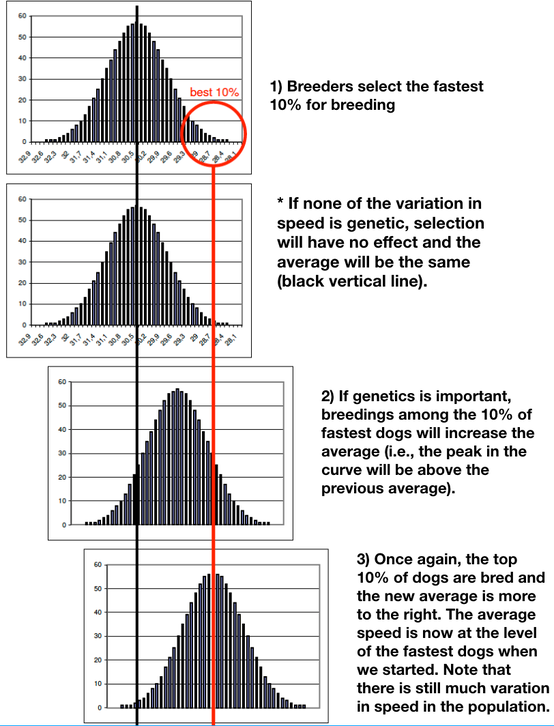
We could mate the fastest male in the litter with the fastest female, and again select the fastest of their progeny.
We might end up with dogs faster than the first two parents, but over the generations of inbreeding and strong selection (breeding only "best to best"), progress will start to level off. We will have eliminated much of the original genetic diversity in the dogs of the first generation. In fact, the puppies in each litter will share a larger and larger fraction of the same genes every generation. We are not going to get faster and faster dogs forever. Why? Apart from the deleterious effects of inbreeding, we have tossed out the genetic variation necessary to produce improvement in a continuous trait by exploiting additive genetic variation. We have bred ourselves into a dead end.
Redefine what you mean by "best". We do definitely want to select the best animals to breed, but we need to think of it in terms of the genetics of the population. To improve on a polygenic trait, we need genetic variation to select for. Instead of selecting a single best dog, we can select a subpopulation of dogs that are all faster than average. We will then use the genetic variation in those dogs, and the random inheritance of that variation in the offspring, to push racing performance in the direction of higher speed.
Can we keep producing faster and faster dogs forever? Probably not. If we don't run out of genetic variation, at some point we will run up against the limits of design and physiology. But we can definitely use selective breeding in this way to improve most traits that are polygenic in dogs.
Note that there is another issue here that breeding in this way addresses. The phenotype of polygenic traits is likely to be a reflection not just of genetics, but also of a roster of non-genetic factors, some of which might be known but most will not. When you select only the "best" individual to breed, you are selecting for the combination of genes and environment that produced what you perceive to be best. But you really only want to be selecting based on genes, because only that part of a trait can be inherited. Maybe the fourth "best" dog has great genes for the trait you want but was raised in a less than ideal situation. That dog's genes will get tossed if you only select the single best animal. By breeding a subpopulation of the top performing dogs, you preserve the genetic variation you need for selection and also reduce the influence of non-genetic factors in your choice.
Let's breed better, healthier dogs. Learn about the quantitative genetics of continuous traits and how you can use this knowledge to your advantage in your breeding program.
Taubert H & D Agena. Quantitative Traits. (no date, no publisher)
Lush, J.L. 1937. Animal Breeding Plans.
|
|
ICB's online courses
***************************************
Visit our Facebook Groups
ICB Institute of Canine Biology
...the latest canine news and research
ICB Breeding for the Future
...the science of animal breeding