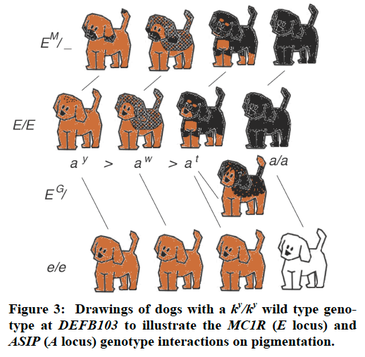
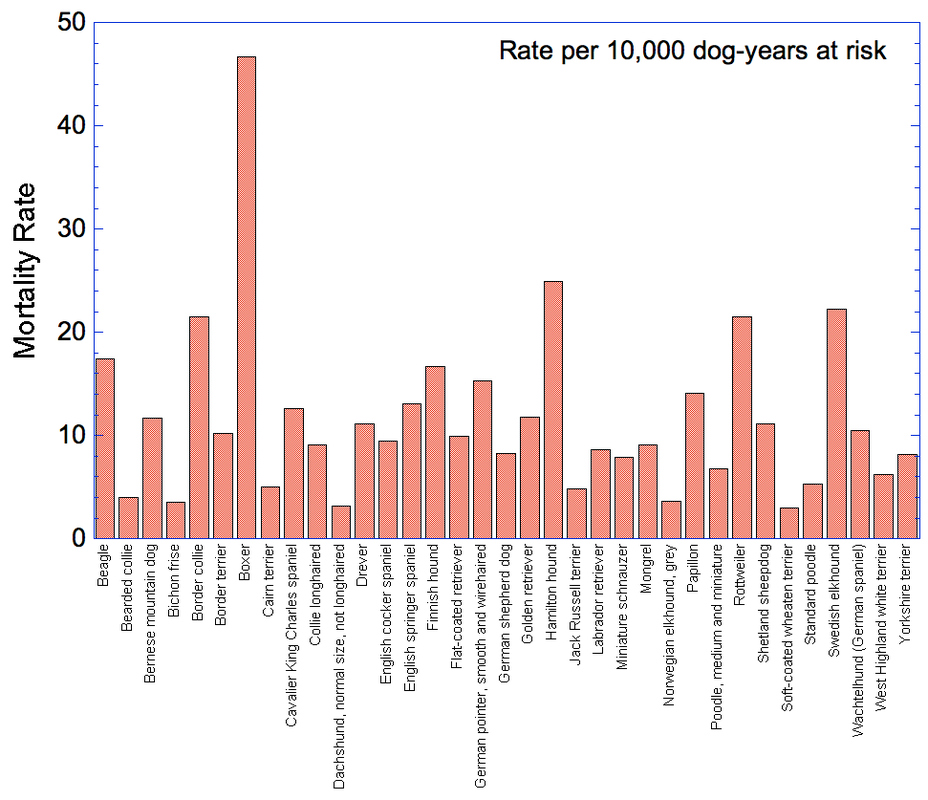
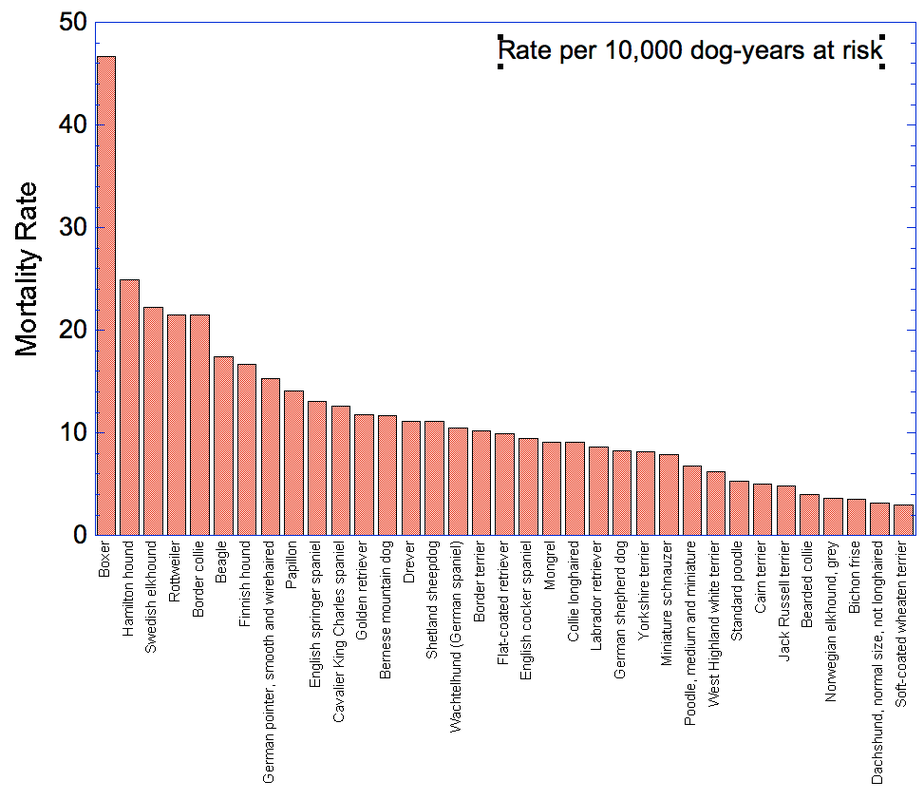
You probably lie awake at night wondering about this. How do dogs get water into their mouth without putting their face in it. And how do they manage to get so much of it on the floor?
Science now knows.
As I'm sure you are aware, dogs can't suck. It has to do with the fact that they have "incomplete cheeks" (yours are "complete"), so they can't pucker up and generate negative pressure to pull water into their mouth. What they do is actually more interesting - they essentially "pull" water into their mouth. They dip their tongue into the water and curl it back on itself forming a cup that contains water, then they draw it quickly into the mouth. This creates a column of water behind the accelerating tongue that also draws the water into the mouth. And they don't do this especially carefully. Cats don't make such a mess because they dip their tongue in the water more delicately. It's finesse, really. (The link above also has a cat video.)
Easier to watch than explain -