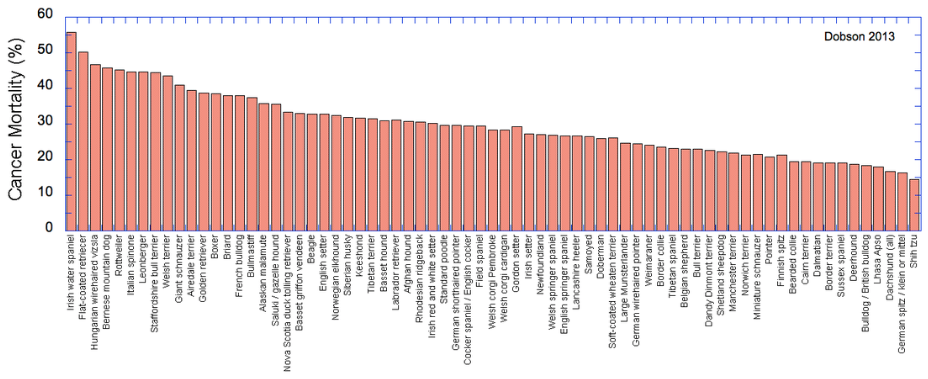
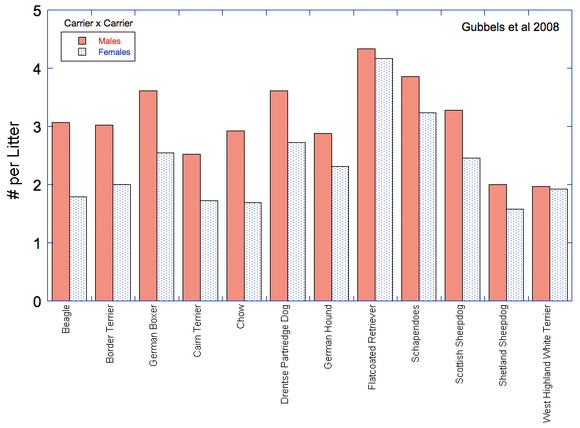
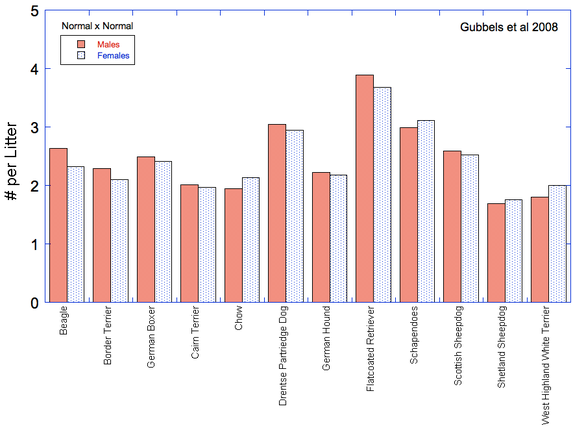
Some breeds seem to have much higher rates of cancer than others. Flat-coated Retrievers, Bernese Mountain Dogs, and Golden Retrievers are a few breeds that seem to be getting hit hard. There are some good breed-specific data on cancer in purebred dogs that I've summarized on the Cancer page in the "Health Data" section of the ICB website, and I've reproduced the relevant summary graph here.
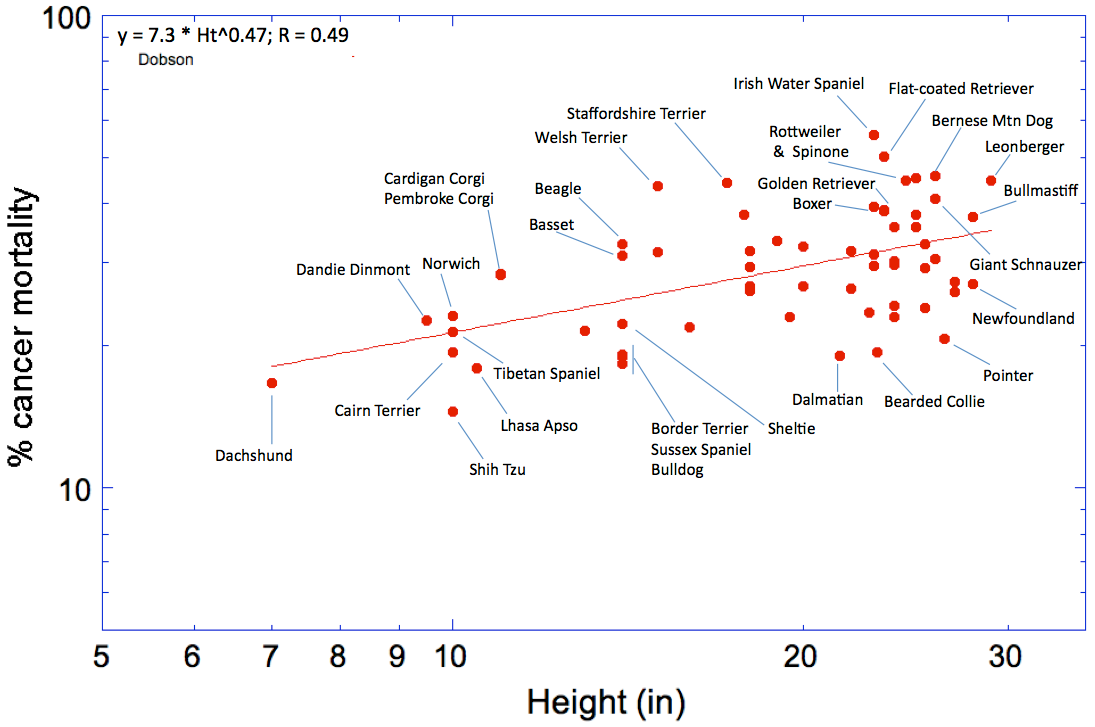
An interesting thing about cancer in dogs is that large dogs seem to have higher rates of cancer mortality than smaller dogs. I took the same data in the graph above and plotted it as a function of height at the withers, and you can see from the resulting graph (below) that mortality from cancer increases with size in dogs. (Note that the axes have been logged so the scale of x and y axes is not linear).
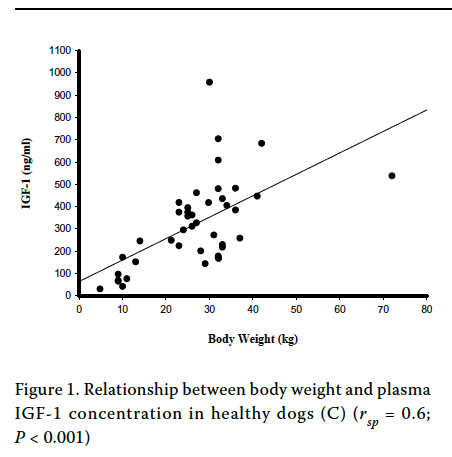
So, are IGF1 levels higher in larger dogs (or lower in smaller dogs)? It seems so (Spichiger et al 2006; Kimberly et al 2011) -
The other interesting thing about my graph of cancer mortality vs height is that there is a lot of scatter around the line. For breeds of a particular height, cancer mortality can vary two-fold or more. For instance, for their size, the Shih Tzu has an exceptionately low rate of cancer, while the cancer mortality of Welsh and Staffordshire Terriers is much higher than you would expect for their size. Among larger breeds, the Irish Water Spaniel, Bernese Mountain Dog, and Flat-coated Retriever are still stand-outs, while the cancer rates in the Pointer and Newfoundland are relatively low.
Tossing all of these numbers onto a graph as we did at the top, you can easily spot the breeds at the high and low ends of the scale. But this doesn't reveal the underlying and very important relationship between cancer mortality and size. It raises two very interesting questions -
1) why do larger animals have higher mortality from cancer, and
2) why do some breeds have much lower rates of cancer than other breeds of about the same size?
Boy, wouldn't we like to know.
Kimberly AG, LM Hughes, & MM Masternak. 2011. Connecting serum IGF-1, body size, and age in the domestic dog. AGE 33: 475-483.
Spichiger, AC, K Allenspach, Y Zbinden, MG Doherr, S Hiss, JW Blum, & SN Sauter. 2006. Plasma insulin-like growth factor-1 concentration in dogs with chronic enteropathies. Vet Med 51: 35-43.
Visit our Facebook Groups
ICB Institute of Canine Biology
...the latest canine news and research
ICB Breeding for the Future
...the science of animal breeding