Population Geneticist
Genetic Counselling Services
The Netherlands
http://www.gencouns.nl/
(Download pdf)
Translations available:
1. Introduction
In the course of the second half of the 19th century the method of ‘inbreeding and selection’ took hold in domestic animal breeding. From 1900 onwards it was widely applied both for companion animals and in agricultural animal husbandry. Especially in the early phases of pedigree breeding (oriented to conformation) the method yielded advantages. With its use breeders were able to ‘fix’ desired traits in their breeding stock and have these transmitted reliably to subsequent generations.

In the world of pure-bred dog breeding ‘inbreeding and selection’ is still the order of the day. What it basically comes down to is this: the level of inbreeding is gradually raised (via line breeding). The purpose is to fix the superior traits of dogs in the line. Selection is in favour of the desired characteristics (the most breed-typical traits), and against undesired characteristics (such as disorders in health and well-being). There are two objectives here: ‘preservation’ and ‘improvement’.
Most breeders are convinced they are ‘preserving’ as long as they mate pure-breds only. As to ‘improving’, most breeders hold that this can be achieved by always letting the ‘best’ animals contribute as much as possible to the next generation. They assume that this approach will also check the frequent occurrence of health and well-being problems in pure-bred dogs. The reality of pure-bred dog breeding, however, yields a different picture.
For the most part the breeding structure of our pure-bred dog populations is very complex. Generations overlap; individual contributions to the next generation keep changing, as do those of lines and selected groups; every breeder has his own selection priorities. In the real situation of breeding practice we cannot isolate the consequences of the various measures of a breeding policy. A whole lot of genetic forces impacts simultaneously. Sometimes those forces are at cross purposes, sometimes they reinforce each other. To gain insight into the consequences of our breeding policy we must look at the effects of every one of our breeding measures separately.
We turn to a model population to learn about the influence and effects of the genetic forces at work. Such forces are of different kinds. Some are laws of nature. They hold for any population, whether we intervene or not. Other forces are triggered by us, processes set in motion by our breeding measures.
We can see what happens when we apply a series of measures to the model population, and ‘translate’ the lessons learned to the reality of breeding practice. Step by step we can introduce complications. At the end of the exercise we arrive at statements about conditions that approximate the actual reality of breeding.
2. A model population
The model we use is the so-called ‘random mating population’. This is an ideal population for which we have formulated clear breeding rules, so that we can demonstrate the separate impacts of breeding measures.
We assume a ‘large’ population. In it, we use enough breeding stock, every generation again, to gather an A-select sample of the available genetic material to transmit from one generation to the next. All of these animals contribute equally to the next generation. Moreover, we exclude other forces that would cause changes in the genetic composition of the population, that is: we do not select, there are no mutations, and we allow no migration. Finally, we exclude inbreeding, meaning that there will be no consciously arranged parent combinations. Mating combinations occur purely at random.
Obviously, in real life there are no such populations, certainly not among dog breeds. This ideal population is fictional and our conditions are strictly artificial.
The Hardy-Weinberg Equilibrium
For a population that meets these conditions the Hardy-Weinberg Equilibrium is applicable: there is a fixed relationship between the gene frequencies and genotype frequencies; these frequencies do not alter in successive generations.
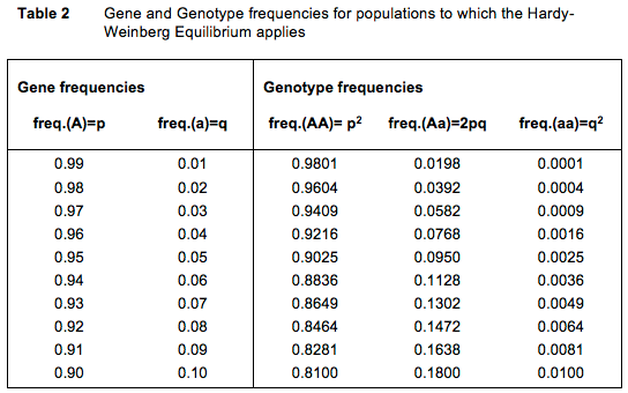
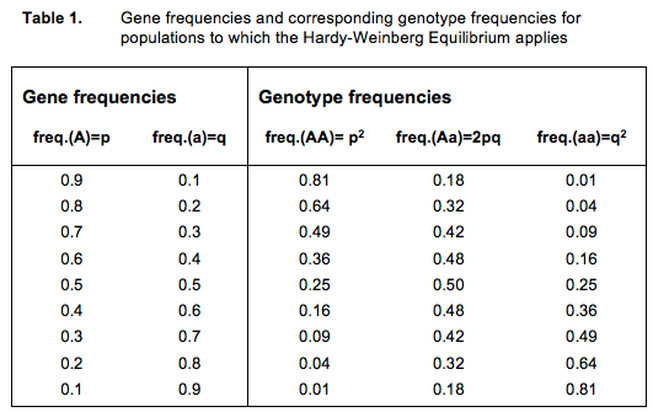
Leaving details aside we can say that: if we indicate the frequency of gene A with p, and that of a with q (where p + q = 1), the frequencies of the genotypes AA, Aa and aa are p², 2pq and q² respectively (where p² + 2pq + q² = 1). We can demonstrate that, as long as we stick to the model conditions stated above, these gene and genotype frequencies will not alter in the succession of generations.
Thanks to the Hardy-Weinberg Equilibrium we know how often the three genotypes AA, Aa, and aa occur in our model population - as long as we know the gene frequencies. Table 1 presents the genotype frequencies for a number of combinations of p and q. For A and a we can take any arbitrary gene pair.
Suppose that in our model population the frequency of the gene for black coat (A) is 0.9, and that hence the frequency of the gene for brown (a) is 0.1. This means that 81% of the animals of the population is AA, 18% is Aa, and 1% is aa (see Table 1, first row).
Since black is dominant over brown, we cannot distinguish the homozygotic black dogs (AA) from the black dogs carrying brown (Aa). We notice that we have a population consisting of 99% black dogs (AA and Aa) and just 1% of brown dogs (aa). However, we know that 18% of the blacks (one in 5 or 6) carry the gene for brown (a) and can transmit the gene to half of its offspring.
As long as we talk about the coat colours black and brown this is not very exciting. The coat colours black and brown contribute little to the health and well-being of the dog, even if the dog’s colour may contribute to the well-being of the owner.
Suppose that in our model population we have a form of cataract with a single-gene recessive mode of inheritance. Let A stand for ‘healthy’ and let a refer to the gene for cataract. In our model population we have only two kinds of dogs: healthy dogs (AA and Aa), and dogs that suffer from cataract (aa). Suppose also, that a survey has shown that 4% of our dogs suffer from cataract.
Because this is a model population in which breeding is entirely according to the stipulated conditions, we are able to say more about the occurrence of cataract in this population. In Table 1 (second row) we can see that the gene frequency for cataract (a) is 0.2. For breeding practice this is not very interesting. We cannot see genes or gene frequencies, and we cannot plot a direct course on this in our breeding. On the other hand, very important to recognize for our breeding is that 32% of the dogs in the population carry the harmful gene, and every single one of these can transmit the harmful gene to half of its offspring. That means that one out of three ‘healthy’ dogs is carrier (Aa) for this hereditary disorder. And that spells a sizeable problem: all ‘healthy’ dogs are equally healthy and, without additional investigation (tests) we have no way of distinguishing between AA and Aa dogs. Basically we have to assume that every healthy dog may well be a carrier.
In every real-life breed we encounter a large number of deviations (harmful genes) each of which occur in low to very low frequencies. Because they occur but seldom, we believe we have little reason to worry. These are deviations that occur so rarely that in fact almost nobody knows them first hand from their own breeding.
It is worthwhile to see what the Hardy-Weinberg law teaches us about the presence of ‘carriers’ when we are faced with rare disorders (Table 2). The general feeling is that genes for rare disorders hardly occur in the population, and that we do not really have to take them into consideration.
Suppose we have a deviation that occurs only once per 10,000 individuals. Table 2 shows that in that case almost 200 carriers per 10,000 individuals are born. Very concretely this means that nearly 2% or about one in 50 dogs, carries the harmful gene.
The incontrovertible fact is that every individual carries the genetic predisposition for a wide range of deviations and disorders. We cannot prevent that. But we can influence the degree to which progeny is born that suffer from hereditary deviations and disorders. This depends on the choices we make in our breeding policy. Below we will illustrate a number of the choices we can make.
3. Selection against hereditary deviations and disorders
As soon as we are confronted with a genetic problem the most logical step is to select against it. Via our selection we exclude from breeding dogs that carry undesired genes. In this way we lower the frequency of those genes in the group of animals we use as parents. Thus, we reduce the risk that in the next generation animals are born that suffer the same ailment. Selection is a breeding instrument by which we can alter the genetic composition of populations. We will demonstrate this with examples (see Table 3).
Suppose that in our population 4% of the dogs suffer from cataract. Once again we are dealing with the model population described above, large, random and so on.
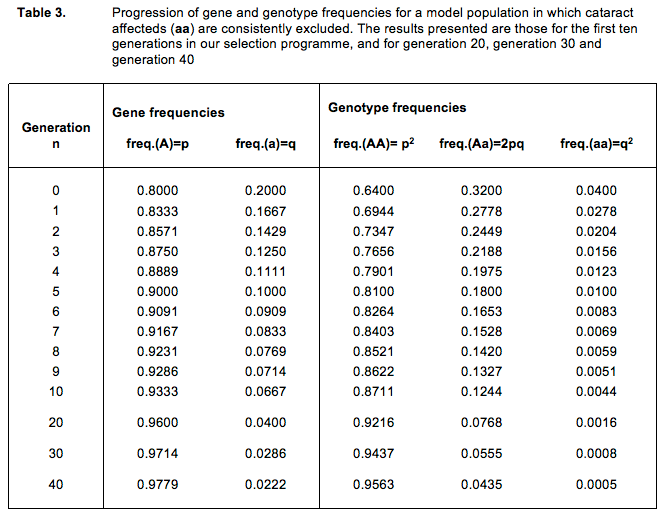
From now on however we decide that we are going to select against this disorder and we do this by excluding dogs having cataract (affecteds, aa-dogs). If we keep excluding aa-dogs we lower the frequency of the a gene every generation again, so that every subsequent generation gives birth to fewer cataract affecteds. Let us discuss a number of steps in this selection process.
When we begin our selection (generation 0) we have 4% affecteds (aa animals), all of whom we exclude from breeding. For the next generation (generation 1) we use only the ‘healthy’ animals (AA and Aa). This means that the gene frequencies of the generation-1 parents are different from those of the generation-0 parents. We exclude all affecteds of generation-0, and so prevent a batch of a genes from being passed on to the next generation.
In generation 1 the percentage of cataract affecteds (aa) born is much less: 2.78% (see Table 3, second row). Again, we exclude affecteds from breeding. To obtain a next generation (generation-2) we use ‘healthy’ animals (AA and Aa) only. And again, excluding the a genes of the affecteds means that the gene frequencies of the parents of generation-2 are different compared with generation-1 parents.
In generation-2 therefore only 2.04% affecteds are born. We keep repeating the process in this and all following generations; every time we exclude affecteds and breed with ‘healthy’ animals only.
Our selection programme marches on marvellously. After two generations we already halved our problem and in the fifth generation the percentage of cataract affecteds born is just 1%. After 10 generations the frequency of affecteds is brought down to 44 per 10,000 dogs (less than 1/2 %). After 20 generations we have only 16 affecteds per 10,000 dogs, after 30 generations we find 8 affecteds per 10,000, and after 40 generations we are left with only 5 individuals suffering from cataract in 10,000 dogs (0.05%)! In short, our programme of selection is a resounding success, at least, in terms of reducing well-being problems in our dogs.
There is a second reason to be modest and reserved about the success of our breeding programme. After 10 generations we still have over 12% of ‘carriers’ (Aa) in the population, and after 40 generations (a full century) the proportion of carriers is still over 4%. Even if in the course of our selection programme we see a dramatic drop in the percentage of affecteds we did not succeed in getting rid of the harmful gene. It remains very much present in all those invisible and hence unidentifiable carriers.
We are forced to conclude that we will in fact never do away with the harmful gene. It will continue to lurk in the population’s carriers. This confirms our earlier proposition to the effect that genetic disorders are part and parcel of life.
4. Over-use of breeding stock
Against the background of the above we run into another problem. The tendency in modern breeding is to make much use of dogs (especially males) that score well in terms of conformation or performance. These are dogs that display outstanding breed-typical qualities, and meet the health requirements as stipulated for the breed. These dogs, it is believed, will provide essential contributions to further development (improvement) of the breed. The idea is that their ‘superior genetic make-up’ should be spread throughout the population with almost no restriction.
The problem that these dogs have in common with any other dog (or any other mammal) is that they are carriers for a large number of genetic deviations and disorders. Mind you, as far as we can see they are entirely healthy. Nevertheless, like any other individual, they bring with them the usual genetic load. For every characteristic of which they are ‘carrier’ they will pass on the harmful gene to half of their children. These are dogs that, just like any other dog in the population, can cause a breed-specific problem if we make disproportionate use of them. A few examples will show us how it works.
For the sake of clarity we will again limit ourselves to one pair of genes. Let’s go back to our selection programme against cataract. We introduced selection in our model population, and so managed a drastic reduction of the percentage of cataract affecteds. An important condition in our model is that for every successive generation again we rely on a large number of breeding animals, all of them contributing to the next generation to the same degree.
Suppose now that we discover a male dog that, we are truly convinced, possesses so many superior traits that we really think this male should have more progeny than a run-of-the-mill dog in the population. After some deliberation we decide that this extremely beautiful or top-performing male should sire 10% of litters in the next generation. After all, this is a rare, ‘once-in-a-lifetime’ dog, and we are prepared to relax just once the strict breeding discipline we agreed on when we began breeding in our model population.
Suppose, next, that we are unlucky. It so happens that our superior sire turns out to be carrier for the cataract gene (Aa). This is not really far-fetched. We have seen that even after a hundred years of selective breeding one in 25 dogs is still carrier. What is the effect of our decision regarding this male in our agreed-upon selection program against cataract?
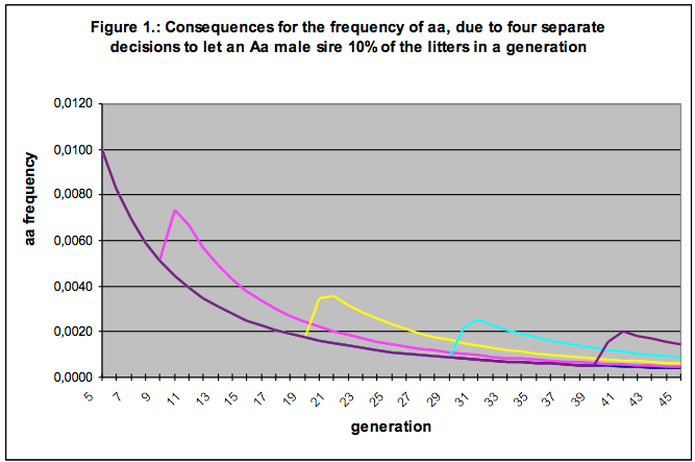
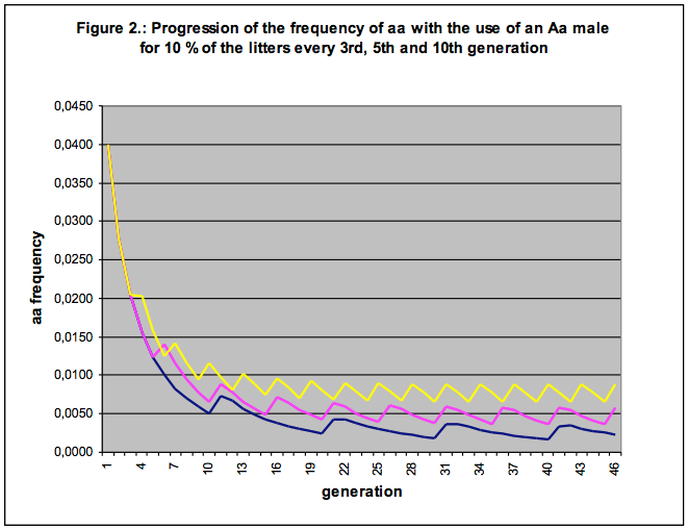
Figure 1 presents five distinct situations. We start out from the selection programme as given in Table 3, and follow the segment of affecteds (aa) from the level of 1% in the fifth generation.
The lower, gradually decreasing curve shows the result of our selection program when we strictly maintain our agreed-upon policy, i.e., exclusion of affecteds and proportional use of all breeding animals.
In addition, we pictured four situations where we trace what happens if we assign 10% of our litters of the 10th, the 20th, the 30th or the 40th generation to a carrier male (Aa). This Aa sire will pass gene a on to half of its children and so increase the occurrence of affecteds (and carriers) in the population.
Each of the four examples represents one violation of our breeding agreements for the model population, other than that we stick to the rules and continue our selection programme.
The results as sketched in Figure 1 actually picture exactly what we wanted to happen, namely, that the genes of this male would impact on the population. Except that we hoped to restrict this to all those good genes that male carried. It was, we said, a male with outstanding traits, and we made extra room for him in our breeding precisely because we wanted to spread his excellent qualities throughout the breed, not realising that the very same thing would happen with the ‘bad’ genetic predispositions that any dog is sure to carry along with the good.
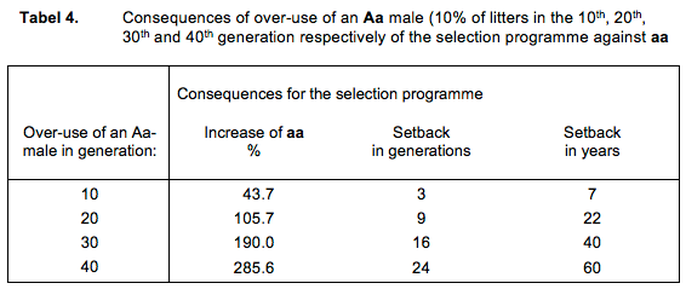
Table 4 summarises what Figure 1 shows us. Over-use of the one male has consequences for the genetic composition of the population. Due to the use of an Aa male in the 10th generation the percentage of affecteds rises by more than 40%; our selection programme experiences a setback of about 3 generations and thus we lose the equivalent of more than 7 years of selection activity.
In the above example we assumed that we had ‘bad luck'. The one time that we felt called upon to bend our breeding rules for the model population it transpired that the chosen superior male ‘happened’ to be a carrier (Aa). According to the laws of chance the dog was far more likely to be free of cataract (AA).
For this one harmful gene the reasoning was sound: in the population we had ‘only’ four, seven or twelve per cent carriers, and in our example the fates surely visited us. On the other hand, every dog carries some dozens of harmful genes and even if the male had been free of the genetic disposition for cataract he was still carrier for a large number of other harmful genes.
Critics are likely to argue that our dog populations are inbred to the point that a large part of the original genetic diversity is lost. That means that not only many of the ‘good’ genes are gone, but a large part of the bad and harmful genes have disappeared as well. This is true. The process of diminishing genetic diversity due to inbreeding does not distinguish between desired and undesired genes. Reality however teaches us that in all our breeds we are still left with so many undesired genes that getting rid of some of them leaves us with no extra room for breeding. What actually happens is the opposite. Due to progressive loss of genetic diversity the population slowly loses vitality. This leads to more rather than fewer hereditary problems in the breeds: increasingly we are faced with groups of deviations and disorders, with complex modes of inheritance, that formerly occurred rather rarely. Over-use of breeding animals, including “top-sires”, is one of the main causes of genetic loss.
Let us turn again to the mutually opposing forces of selection and over-use. In Figure 1 we have seen what this might mean for a hereditary deviation that hardly ever occurs. Say we have a very rare disorder in our breed. It occurs only in 5 out of 10,000 dogs and, for 10% of the litters of the next generation, we decide to use a male that, as it turns out, carries this rare gene.
This is the situation we encounter in the 39th and 40th generation in Figure 1. If we really were to let this dog sire 10% of the 40th-generation litters we would cause a rise in the percentage of affecteds that would saddle us with sixty more years of consistent selection to restore the old situation.
Compared to the reality of present-day dog breeding the chosen example is extremely mild. The truth is that, especially in numerically small breeds, a significantly higher over-use of males is applied than the 10% chosen here. To this we must add that, since these are dogs of which everybody has high expectations, large numbers of their progeny tend to be used for breeding as well. Consequently, the percentage of affecteds will often climb far more than Figure 1 has it and in the generations to follow the rate keeps going up. In many breeds we have seen examples of this. ‘Suddenly’ a hereditary deviation that hardly troubled us explodes into a ‘breed-specific’ problem.
There is a second reason why we can call the example very mild. We spoke of a ‘once-in-a-lifetime’ sire, a dog of exceptional quality such as we are not likely to meet again in a hundred years. But in dog breeding over-use is the rule rather than the exception. ‘Once-in-a-lifetime’ sires are emerging every year. The whole of the breeding exercise is oriented to using too small a number of usually close kin to produce the next generation, most of which (especially males) supply a disproportionately large contribution to the breed. A fair representation of genetic material is in no way transferred from one generation to the next. On the contrary, over-use has become the basis for breeding pure-bred dogs.
To let the model approximate a little more the actual practice of dog breeding, let us see what impact repeated over-use has in relation to our selection programmes. In Figure 2 we compare the progression of the frequency of dogs suffering from some genetic deviation (affecteds, aa) in three situations. Once again we start from our model population (Table 3) in which we select against affected dogs and assign 10% of the litters to an Aa sire in every 3rd, 5th or 10th generation.
Selection is an important instrument in breeding. But with our selection we are waging a lost battle against the impact that over-use has on the genetic composition of the population. There are plenty of examples of this in the actual practice of breeding. In spite of all selection efforts breeders are often unable to really reduce the level of deviations in their breed.
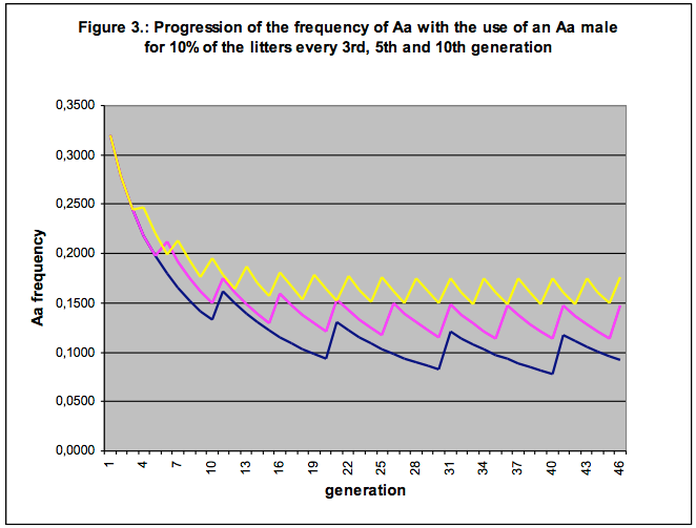
The breeding method as applied in fact draws us into a kind of vortex. The percentage of affecteds (aa) keeps increasing because every generation again we breed with too few animals. On top of this the percentage of carriers (Aa) keeps increasing too, to the measure we diverge from the breeding rules of the model population more often and in more ways (see Figure 3).
In the selection programme (Table 3) we saw the percentage of carriers between the 20th and the 40th generation diminishing from 7.68 to 4.35 per cent. But in the examples chosen in Figure 3 we have to accept an average percentage of carriers of 10 per cent to more than 15 per cent. This means that the risk we run of using a carrier is also two to three times greater.
And so the vortex sucks us down, inexorably, unless we take measures to redirect the breeding management of our dog breed populations. We cannot go on with our ‘repair policy’, persistently introducing still more expensive selection programmes to keep health and well-being problems within bounds. It has not helped us to systematically reduce the problems to an acceptable level. Moreover, on the level of prevention we neglect to do almost everything that we could do to forestall problems. Even if we want to believe that in technical and business-economic terms the current breeding method is still acceptable, it is certain that from the point of view of animal well-being our present breeding method has long ceased to be justifiable.
5. Expansion of scale in breeding
When, a hundred years ago, breeding pure-bred dogs took hold, we were dealing with a small-scale breeding structure. Breeders applied ‘inbreeding and selection’ and built their own inbred line. ‘Over-use’ was the order of the day, but it was small scale and mostly restricted within the lines of individual breeders. As soon as problems emerged in their line that could not be overcome via selection, the breeders would turn to a fellow breeder to introduce ‘new blood’.
Translated into terms of breeding: as soon as the level of inbreeding became too high, so that little room was left for selection, breeders restored part of the genetic diversity by crossing in more or less unrelated animals. They sacrificed some of their line’s homogeneity, repaired part of the selection room and were then able to continue their ‘inbreeding and selection’ again.
In the early days, when breeding was still modest in scale, this breeding method was satisfactory. Every time a breeder ‘ran stuck’ with his own line he could find another breeder from whom to obtain breeding stock that was sufficiently unrelated to his own line. This went awry after World War II, when society became increasingly mobile. It became a whole lot easier to move around and to come into contact with distant breeders. Breeders acquired the ‘new breeding stock’ they needed far away from home. But this also meant that increasingly breeders made use of the same (champion) lines and that slowly but surely the mutual kinship between the distinct lines grew. In most breeds we see that this process has accelerated over the last decades and that breeds are gradually turning into one large single inbred line. The breeder whose line experiences problems pays the price. In search of breeding stock unrelated to his own, to restore some genetic diversity, he has nowhere left to turn.
Of course, the first to pay the price are the dogs. When the breeder runs into problems there is no solution for his line (for his dogs) to keep health and well-being problems in hand. Over-use of breeding animals and lines produces dogs that possess the same genetic predisposition, deriving from the same shared ancestors. As we saw above, the effects of this on the genetic composition of the population can no longer be controlled through selection. And so it is that genetic problems become breed-specific traits.
In recent health surveys it became painfully clear that most of our dog breeds are struggling with an unnaturally high level of genetic deviations and disorders. In other animal species we express the frequency of genetic disorders almost exclusively in per mil (or even fractions of per mil). In pure-bred dogs we are forced to express disorder frequencies in terms of percentages and multiple percentages.
How this came about we can understand very clearly from the history of pedigree dog breeding. We understand, but need not accept. Our joint responsibility for our breeds dictates that we must find solutions.
Throughout the past century population management and breeding policy for almost all breeds was established as the sum total of individual breeder decisions. Because there was no policy for the breed as a whole it happened that breeders made choices that for their own line were often quite defensible and correct, but proved harmful for the breed. Here lies the root of the current health and well-being problems in our pure-bred populations.
6. A new breeding policy
Above, we tried to demonstrate in a number of steps the consequences of our breeding decisions. We did this with the help of a model population in which the effects of our breeding decisions were visualised. In this way we could get around the complexity of ‘real life’ and see what genetic forces actually come into play when we take specific breeding decisions.
We first focussed on ‘preservation’, the basic objective of every breed club. In the model population (random mating) we preserve the gene pool and hence the characteristics of the population (the breed). Preservation is assured because every generation again we transfer a sufficiently large sample of the genetic material to the next generation. Moreover, we ruled out all forces that could alter the genetic composition of the population. This model population therefore remains constant; it does not ‘improve’ nor does it ‘deteriorate’. The traits occurring in the population will resurface with the same frequency in each new generation. Actually, this is not very satisfactory; in every real-life population there are aspects that we might want to improve on. Maybe we’d like to make our dogs still more ‘breed typical’ and certainly we would prefer to breed without any genetic health and well-being problems at all. Especially this second wish is, in light of current social developments, the more relevant and pressing.
The breeding instrument to alter a population (a breed) is selection. Selection is the way to deal with shortcomings occurring frequently, but the closer we come to our goal the less effective selection will be. In the course of the selection process the relation between the part that can be controlled (the affecteds) and the part that cannot (the carriers) becomes in fact more unfavourable in each successive generation. Selection will never enable us to get completely rid of a genetic problem. At best we can bring down the occurrence of disorders to a biologically attainable and morally acceptable level.
Traditionally, breeders applied inbreeding (line breeding). Initially inbreeding was a breeding instrument applied small-scale, and the breeds could be characterised as a pluriform collection of inbred lines (one look at a dog told you what kennel he came from). In our efforts to achieve our breeding goals more quickly (our ‘improvement drive’) we have in the past years turned more and more often to fewer and fewer breeding animals. We were not satisfied with good representatives of the breed, we wanted the best. Because everyone wanted the best, and because everyone agreed on which animals were the ‘best’, we ran into the phenomenon of ‘over-use’. We turned a lot of breeds into a single inbred line where all animals have a shared ancestry. All this exacted its toll from the original genetic diversity in the breeds; ‘preservation’ was squeezed.
Breeders overlooked that in tandem with the spread of desired genes the unwanted genes were being spread apace. The result that literally every animal is carrier for dozens of harmful genes was neglected. Breeders believed that they could stay on top of the problems by selecting against undesired traits. Selection however will not reverse the effects of over-use and concomitantly rising inbreeding levels throughout the population. The above has demonstrated this.
Our breeding has now come to the point that we have to make choices. If we carry on with our current breeding policy, the continued existence of a large number of breeds is at risk: we will simply be unable to contain the rampage of genetic problems. It is at population (total breed) level that we must arrive at agreed-upon rules intended to halt the development of past decades and next to turn these around. Very concretely this means:
1. We must attune the use (the contribution to the next generation) of breeding animals to the size of the population. No single dog should have an impact on the genetic composition of subsequent generations such that ‘genetic disasters’ can arise.
2. If we do this we can once again make effective use of the old method of ‘individual selection’, and take the first steps towards genuine improvement of the health and well-being condition of the breeds.
3. We will have to provide breeders with instruments that allow them to control the level of inbreeding in their lines. The use of inbreeding can be advantageous in breeding, but it must remain an instrument rather than turn into an irreversible and unavoidable force.
4. Over and above the individual selection that has been applied since 1900 we must make modern methods of selection available for dog breeding (breeding value estimates, genetic risk assessments).
In short, this means that we must first make sure that we do not add to our problems, that we use currently available methods to work on improvement, and that we must make haste to provide breeders with modern instruments to guide breeding and to combat problems in our dogs. It is only then that we can justifiably talk about responsible genetic management of our pure-bred dog populations.
Source:Centennial Conference of the Dutch Kennel Club, 2 July 2002, Amsterdam, The Netherlands.
(Download pdf)
If you found this to be useful, please consider supporting ICB by becoming a Member.
And join the discussion in our FACEBOOK GROUP - ICB Breeding for the Future